BKC1253: Physical Chemistry Assignment, UMP, Malaysia A researcher wishes to determine the molar mass of a metal and a nonvolatile solute. For the metal determination
| University | Universiti Malaysia Pahang (UMP) |
| Subject | BKC1253: Physical Chemistry |
Question 1
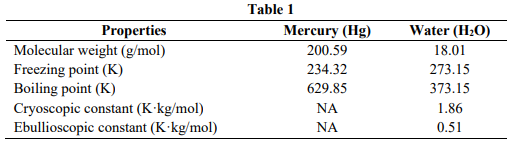
A researcher wishes to determine the molar mass of a metal and a nonvolatile solute. For the metal determination, he dissolves 1.2 g of metal in 100 g of mercury and then the mixture is heated to boiling. For the nonvolatile solute, he dissolves 3.8 g of solute in 300 g water. The properties of mercury and
water are stated in Table 1.
- Determine the molar mass of the metal if the partial pressure of mercury vapor over the boiling mixture is 754 Torr and the vapor pressure of pure mercury is 769 Torr.
- Determine the molar mass of the nonvolatile solute if the mixture freezes at 272.50 K.
Question 2
It is intended to mix gas oxygen and gas nitrogen at a temperature of 28 °C. If the mass of gas oxygen is 140.8 g and gas nitrogen is 436.8 g, determine the change in molar Gibbs energy (in joule unit) when these gases mix. Is the mixing spontaneous?
Question 3
The formation of hydrogen iodide could be obtained from a reversible reaction of hydrogen and iodine as H₂ (g) + I₂ (g) ↔ 2 HI (g). Table 2 shows the thermodynamic values at 298.15 K for the components involved in the reaction. Assume that the vapor behaves as a perfect gas.
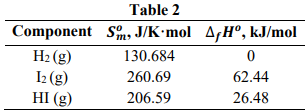
- Determine the values of the equilibrium constant at 298.15 K.
- Determine the values of the equilibrium constant at 723.15 K.
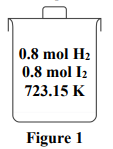
A mixture of hydrogen and iodine is placed in a sealed container as Figure 1. Calculate the amounts of all components in the mixture at equilibrium.
Get Help By Expert
Seeking Budget-friendly Assistance for BKC1253: Physical Chemistry Assignment? Assignment Helper My is your go-to solution. At Universiti Malaysia Pahang (UMP), our proficient experts offer cheap assignment help tailored to your needs. Rest assured, we deliver plagiarism-free and well-researched solutions to ensure academic success. Besides, this our experts provide you top Programming Assignment Help.
Recent Solved Questions
- MPHR7113 Assignment: Strategic HR Analysis of Golden Heritage Café (HRM)
- International Marketing Planning Assignment Malaysia
- JUS101: Teras Keusahawanan Assignment, USM, Malaysia Kursus keusahawanan bertujuan memberi pendedahan kepada pelajar mengenai bidang keusahawan dan
- LAW 3711: Equity & Trusts II Assignment, IIUM, Malaysia Mee Lan left RM 3000 for the maintenance of aged and poor people at Gunung Ledang
- Bachelor of Economics Assignment, HU, Malaysia Students are required to look through the given articles in HLMS and choose one of the topics which they are comfortable
- BBRC4103: Discuss the differences between a management problem and a research problem: Research Methodology Assignment, OUM, Malaysia
- MAT1830: Discrete mathematics for computer science Assignment, MUM, Malaysia The Corruption Perceptions Index uses perceptions of the general public, business people
- Electrical Technology Assignment, UKM, Malaysia Analyze the circuit using, the nodal method, and find the value of voltages at nodes A, B, and C
- SQG2613: Research Methodology Research Paper, UTM, Malaysia Investigate current study trends based on the selected study areas related to Civil Engineering
- MKTG3040: Services Marketing Report, UON, Malaysia You are required to select an experience when visiting a service organisation by choosing any ONE company from ONE category of the industry


